Addressing rouging on stainless steel
Rouging refers to the unwanted red discoloration of stainless steel when exposed to high purity water, as depicted in images 1 and 2. This phenomenon occurs in high purity deionized(WFI) water above 60℃ and also in clean steam. WFI stands for 'Water For Injection' and CS for 'Clean Steam'. Such water is essential for producing certain pharmaceutical products introduced into the human body, among others. Rouging is typically a problem for pharmaceutical companies and, to a lesser extent, for companies in the food sector that utilize 'polished water'.
Ko Buijs - Innomet Consultancy BV
(artikel ook beschikbaar in het Nederlands)
These waters have a high ion vacuum due to their exceptional purity. The ability of this water to dissolve metal ions is so significant that metal atoms can migrate through the dense chromium oxide layer towards the high purity water. This movement is called diffusion and sometimes migration. This 'hunger' to absorb substances is why this water should never be consumed, as it can be life-threatening for humans and animals. Equipment like fermenters, batch tanks, mixing vessels, pumps, valves, and associated piping systems are particularly susceptible to turning red due to exposure to this water at elevated temperatures. The chemical mechanism of rouging development is as follows:
. Fe → Fe2+ + 2e
. 2Fe + 3H2O → 2Fe(OH)3
. 2Fe(OH)3 → Fe2O3.H2O + 2H2O (above 60℃, it generates a red color).
Austenitic stainless steel AISI 316L (1.4404) is primarily composed of iron, which tends to react with water to form iron hydroxide, a basic substance. Initially, the iron enters the water as an ion, releasing two electrons per atom. Above 60℃, this iron hydroxide converts into iron oxide and binds to a water molecule, creating an insoluble compound that deposits, turning the surface red. Since it is insoluble in water, this iron compound is technically no longer part of the WFI-water. However, practical experience shows that it is unrealistic to expect all iron oxide to precipitate 100% to form a layer of rouging. It is plausible that very small oxide particles are still carried along in the system.
Food & Drugs Association
The FDA, or 'Food & Drugs Association', tolerates the rouging issue. Once a solution is found, the FDA will likely prescribe or implement this method, as rouging is a significant concern for the FDA. Many scientists have tried to map out this problem to find a solution for this undesirable phenomenon. They have explored higher alloying of stainless steel, such as using duplex or 1.4435 instead of 1.4404 and 904L (1.4539), as well as the potential presence of delta ferrite. Ultimately, none of these variables have contributed to permanently eliminating the problem. However, electrolytic polishing has shown some positive effects, primarily due to the more resistant oxide layer that is richer in chromium and nickel. This is because iron dissolves more easily during this process than nickel and chromium. Additionally, the surface size is significantly reduced as the 'mountains and valleys' disappear. This results in less interaction with the surface, leading to reduced rouging.
Migration of more elements
A common misconception is that only iron ions end up in the WFI-water, leading people to believe the water does not contain other harmful elements. However, measurements have shown that not only iron ions but also ions of chromium, nickel, and molybdenum end up in this water. These elements do not deposit but are part of the WFI-water. Incidentally, these quantities are very small, so there is no reason to panic. However, it appears that after CIP cleaning of these systems, heavy metal ions still remain in the WFI-water. This is, of course, an undesirable situation. Moreover, these ions are not visible and will therefore simply remain present in the WFI-water. After six weeks of testing in WFI-water of stainless steel type EN 1.4435 in the presence of air containing 1% carbon dioxide and nitrogen, the following quantities of ions were found to be present:
• 40 - 130 µgrams of iron per litre;
• 9 -23 µgrams of nickel per litre;
• 9 µgrams of molybdenum per litre;
• 1 µgram of chromium per litre.
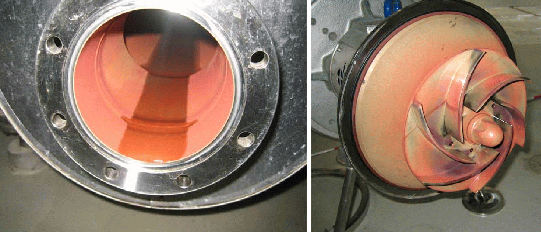
Figure 1: Rouging in a stainless steel AISI316 pipe and on a pump impeller (photos Henkel).
The diffusion of the elements mentioned is shown schematically in figure 3. The blue area represents the WFI-water. It can be seen that the oxide skin consists of five different layers that are a few nanometres thick. Each layer has its own specific chemical composition. The top two layers do not contain any iron compounds. If one analyses stainless steel provided with rouging, it appears that iron atoms have ended up in the top two layers. This is proof that iron migration has taken place. The oxide skin is normally 10 – 15 nanometres thick and if there were no iron in the top layer, there would also be no chromium/iron ratio as can be seen in figure 4. For information, a nanometre is 10-9 only a thickness of a few atomic layers.

Image 2: Rouging on a stainless steel component (photo Henkel).
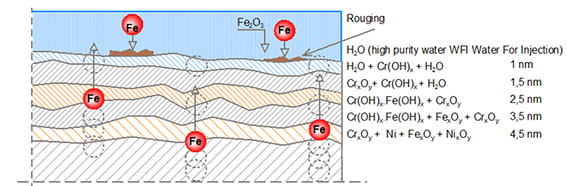
Figure 3: Schematic representation of the diffusion of iron atoms from the stainless steel through the 13 nm thick oxide skin (drawing Innomet Consultancy Ltd.)
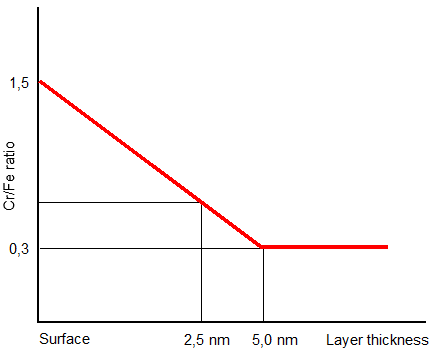
Figure 4: The chromium/iron ratio in the first 5 nanometres of the top layer of an oxide skin.
That is why it is relevant that the problem of rouging becomes a thing of the past, because although the iron can do little or no harm because it largely deposits, one does have to deal with other metal ions that do not belong in this water. Pharmaceutical companies remove rouging and that is called derouging. This can be done both mechanically and chemically. The basic iron-containing deposit is relatively easy to remove by mechanically wiping the surface, but that is labor-intensive. Chemically, this can be done with an acid, which will cause the following chemical law to occur. It is: base + acid → salt + water. This is also a time-consuming process followed by intensive rinsing. This also means that no pharmaceutical products can be made in the installation in question during derouging. In other words, such activities lead to loss of income.
Determining and removing rouging
In practice, so-called rouge meters are available that indicate when it is time to derouge again. Such meters are usually placed in the pipe and measure the intensity of the red colouring by means of reflection. It often turns out that derouging must be done annually, although it also happens that this must be done every six months. Instead of a rouge meter, a clean white cloth can also be used that indicates whether derouging is necessary after rubbing the surface. Nowadays, encouraging results are also achieved with aqueous organic salt solutions to remove the rouging. These are dissolved salts that must be active for many hours at higher temperatures in order to achieve a good result. However, it is more environmentally friendly than using acids. That is why these methods are also called bio-derouging. All in all, it will be clear that the formation of rouge is a very undesirable phenomenon. After intensive research and investigation, several scientists and research companies have organizedinternational conferences to help eliminate this phenomenon. The company Force Technology in Denmark in particular has done a lot of research to suppress the development of rouging.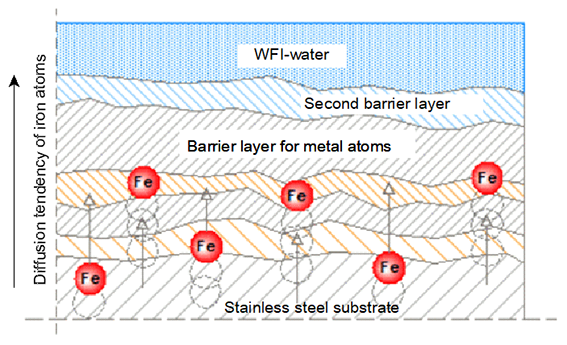
Figure 5: schematic representation of the thickened oxide skin that creates a natural barrier against the escape of metal atoms (drawing Innomet Consultancy Ltd.)
Optimizing the oxide skin
The company Innomet Consultancy came up with the idea of thickening and densifying the chromium oxide skin of stainless steel using atomic oxygen. The aim of this is to substantially increase the barrier to prevent or even completely prevent the escape of metallic elements. This densification can take place both electrochemically and chemically, provided that atomic oxygen is created. Thanks to this densification and thickening of the top two top layers, the metal atoms will most likely become stuck, as it were, so that they remain trapped in the matrix. This enlargement of the barrier is shown schematically in figure 5. The size of the various metal atoms is also shown, which is an indication of the chance that a metal atom can escape from this oxide skin. This is shown schematically in figure 6. The intention is that not only iron atoms are stopped, but also other elements that are present in the stainless steel. Finally, an extensive test program was set up in collaboration with a research company on various samples of stainless steel. In order to create atomic oxygen, ozone, hydrogen peroxide and anodic oxidation were used. Ozone and hydrogen peroxide are unstable compounds that decompose into molecular and atomic oxygen, as well as water and atomic oxygen, respectively. Ultimately, it turned out that all of the media mentioned have the same effect on preventing rouging. In itself, this is logical because all mechanisms release the same atomic oxygen as an oxidizer.
Results of the test program
Testing started with the stainless steel types AISI304 (1.4306), 316L (1.4404), 316L (1.4435), 904L (1.4539) and duplex (1.4462). For this purpose, 56 sample plates were made in a square shape. These plates were suspended in the WFI-water in such a way that a part protruded above the water level. A part of these plates was mechanically ground with K180 and another part was also electrolytically polished after this grinding. These samples were treated with atomic oxygen for 24 hours at a temperature of 20℃. These samples were then partly immersed in WFI-water at a temperature of 85℃ for 2000 hours. The result was that none of the samples showed any sign of rouging, which is of course a very positive and encouraging result.
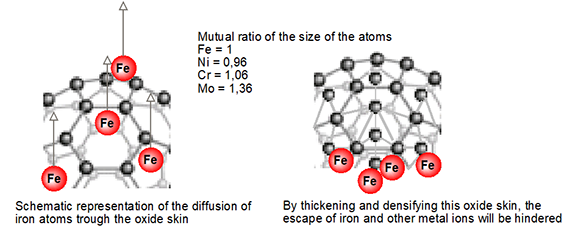
Figure 6: on the left a ‘free passage’ for metal atoms and on the right a barrier due to the thickening and densification of the oxide skin (drawing Innomet Consultancy Ltd.)
Because no rouging occurred at 85℃ it was decided to further increase the temperature to 95℃. These tests in WFI-water at this increased temperature also include a period of 2000 hours. However, after 1000 hours some rouging occurred on stainless steel 304L at the transition between air and water. After 2000 hours all samples showed signs of rouging although sample 904L came out best. Apparently a critical tipping point was reached at this higher temperature. This fact was demonstrated by the development of a new equilibrium in this oxide skin.
Metastability
It turned out that the thickened and densified oxide skin was metastable at 95℃ because the effect of the thickening and densification was lost, which resulted in a normal stable oxide skin. The result was that rouging started to develop again as it usually does with a naturally formed oxide skin. That is why a method had to be found to fix the treated oxide skin in such a way that it would remain stable at the increased temperature of 95℃. So far, no solution has been found for this, but it may be possible by hydrating the surface. The intention is then to fix the thickened oxide skin with demineralized water in such a way that it can form a stable whole. This treatment can roughly be compared to hydrating anodized aluminium. During this anodizing, the oxide layer is made considerably thicker, but that skin is porous on the surface. This immediately gives the opportunity to absorb a dye in this porosity. The action after this is the sealing of the porous oxide layer and that is done with demineralized water. The porous layer absorbs this water and binds to the aluminium oxide, causing it to swell on the surface due to this hydration. In this way, a dense and thick oxide layer is obtained that is considerably more corrosion resistant than non-anodized aluminium. It seems logical that this mechanism could also work with the thickened and densified oxide layer of the stainless steel. Therefore, this could become an interesting research that should eventually lead to a stable thickened and densified oxide layer that once and for all solves the problem of rouging.
Finally
If pharmaceutical companies have systems that do not get hotter than 85℃, it is certainly worth considering treating the stainless steel components with atomic oxygen. There are now clear indications that this oxide layer is stable for a long time, in contrast to an operating temperature of 95℃. As stated above, the treatment with atomic oxygen was carried out at a temperature of 20℃ for 24 hours. It will be worthwhile to have this treatment take place at a number of higher temperatures with variable times. This may also make a positive contribution to the stability of the thickened oxide layer.




